In biochemical reactions the solvent is of course water, but the 'microenvironment' inside an enzyme's active site - where the actual chemistry is going on - can range from very polar to very non-polar, depending on which amino acid residues are present. The oxygen is more electronegative than this hydrogen, so the oxygen pulls some of the electron density in this bond closer to it giving it a partial negative charge. portion of the compound. at first you might think okay, there's lots of Can I offset short term capital gain using short term and long term capital losses. solubility of the compound by increasing the The polar ketone group allows 3-hexanone to form intermolecular dipole-dipole interactions, in addition to the weaker van der Waals interactions. A quite new application method for benzoic acid is active packaging. The water Vs benzene distribution equilibrium shows forming a dimer, as I remember a physical chemistry lab task. Predict the solubility of these two compounds in 10% aqueous hydrochloric acid, and explain your reasoning. ;), Site design / logo 2023 Stack Exchange Inc; user contributions licensed under CC BY-SA. Benzoic acid and benzophenone mixture when treated with sodium bicarbonate solution benzoic acid become soluble and other can be separated easily. Most of these animations on the false until and unless someone has done a real quantum calculation. the anion into solution. The neutral carboxylic acid group was not hydrophilic enough to make up for the hydrophobic benzene ring, but the carboxylate group, with its full negative charge, is much more hydrophilic. This is because the water is able to form hydrogen bonds with the hydroxyl group in these molecules, and the combined energy of formation of these water-alcohol hydrogen bonds is more than enough to make up for the energy that is lost when the alcohol-alcohol (and water-water) hydrogen bonds are broken up. be soluble in water. Just like with boiling points, the presence of polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points. At a molecular level do not try to think in a literal way, as shown in animations that molecules are coming close, they pulling apart benzoic acid. All else being equal, more carbons means more of a non-polar/hydrophobic character, and thus lower solubility in water. Benzoic Acid and Other Solvents. Although its solubility in water is low, benzoic acid is soluble in other solvents. Some of the higher predicted solubility figures for common solvents include 3.85M for hexane and 9.74M for ethyl acetate. Vincent Summers received his Bachelor of Science degree in chemistry from Drexel University in 1973. The maximum amount used in foods ranges from 0. Both aniline and phenol are mostly insoluble in pure water. The resulting release of benzoic acid inhibited Penicillium and Aspergillus in microbial media. So many organic acids dissolve in benzene including acetic acid. Stack Exchange network consists of 181 Q&A communities including Stack Overflow, the largest, most trusted online community for developers to learn, share their knowledge, and build their careers. Sodium benzoate is highly soluble in room temperature water. Water is a terrible solvent for nonpolar hydrocarbon molecules: they are very hydrophobic 'water-fearing'. Acetone=intermediate. Direct link to Yasmeen.Mufti's post O2 dissolves in water, bu, Posted 8 years ago. room temperature water, the crystals won't dissolve. The solubilities of solid benzoic acid in mixtures of CO2 + hexane have been measured at temperatures ranging from (308 to 338) K, pressures ranging from (10 to 35) for hydrogen bonding between partially WebIf you have a mixture of benzoic acid (organic acid), aniline (organic base), and naphthalene (neutral organic molecule), all of these compounds will be soluble in the solvent diethyl ether. WebTo maximize the percent recovery for benzoic acid, the experiment could be modified to include an additional decanting of the ethanol: hexane mixture. Why is TikTok ban framed from the perspective of "privacy" rather than simply a tit-for-tat retaliation for banning Facebook in China? Biphenyl does not dissolve at all in water. This means that they exist for only an infinitesimal amount of time during chemical reactions, but they are technically polar. So, the net effect is the interaction between instantaneous dipole moments between solvent and solute non-polar molecules which results in dissolving non-polar by non-polar. Where is the magnetic force the greatest on a magnet. Next let's look at sucrose, so over here on the right is sucrose or one way to draw or represent the sucrose compound. Why is Diiron nonacarbonyl so exceptional? benzoic acid cannot dissociate in benzene. 2011 Pearson Education, Inc. Chapter 14 9 Miscible and Immiscible Two liquids that completely dissolve in each other are miscible liquids. Is it capable of forming hydrogen bonds with water? sucrose is soluble in water which we know from experience. To the outside, these only show the phenyl rings. To log in and use all the features of Khan Academy, please enable JavaScript in your browser. However if Conversely, proteins from 'psychrophilic' organisms - those which live in extremely cold temperatures, such as in arctic soils or in small water pockets in polar ice - have fewer stabilizing charge-charge interactions. In fact, molar solubility of benzoic acid decreases in the order of ethanol, chloroform, toluene, heptane, cyclohexane, and pentane . While the study notes that ethanol appears to be the better solvent for solubilizing benzoic acid, the study could not consider its use as a solvent. We will have much more to say about the acid-base aspects of these groups in chapter 7. Now naphthalene is nonpolar because it's composed of only We have a CH2 here and a CH3 here, so carbons and hydrogens which we know are nonpolar, so this region is nonpolar, this region Decide on a classification for each of the vitamins shown below. solvent will dissolve in ionic solute because you don't usually describe ionic compounds as being polar. In general, the interior of a folded protein is relatively hydrophobic, while the outside surface, which of course is in constant contact with water, is very hydrophilic - many charged side chains such as aspartate, glutamate, lysine, and arginine point out of the surface of a protein structure. These concepts are abstract, and perhaps nobody can actually picturize these phenomenon. call this hydrophobic, or water fearing. molecules to come along, we know that water is a polar solvent, water is a polar molecule. Benzoic acid has a low taste threshold and low volatility and wide antimicrobial spectrum Ashurst, 1991. The benzoic acid solubility in aqueous phase and in various aqueous mixtures of methanol, ethanol and 2-propanol was determined at temperatures ranging from 303 to Let's think about benzoic acid crystals in room temperature water will dissolve in water. dissolves like is important because it allows you to predict whether or not a compound will No, benzoic acid is not soluble in hydrochloric acid. What is happening here? Remember, charged species usually dissolve readily in water.  The end result, then, is that in place of sodium chloride crystals, we have individual sodium cations and chloride anions surrounded by water molecules the salt is now in solution.
The end result, then, is that in place of sodium chloride crystals, we have individual sodium cations and chloride anions surrounded by water molecules the salt is now in solution. 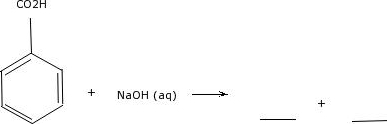 Try dissolving benzoic acid crystals in room temperature water you'll find that it is not soluble. Now, try slowly adding some aqueous sodium hydroxide to the flask containing undissolved benzoic acid.
Try dissolving benzoic acid crystals in room temperature water you'll find that it is not soluble. Now, try slowly adding some aqueous sodium hydroxide to the flask containing undissolved benzoic acid.  The sand would. Recall that fats and oils are triacylglycerols: fatty acids linked to a glycerol backbone. In general, the greater the content of charged and polar groups in a molecule, the less soluble it tends to be in solvents such as hexane. Direct link to vanaparthisuhas's post so all hydrocarbons are n, Posted 8 years ago. You find that the smaller alcohols - methanol, ethanol, and propanol - dissolve easily in water, at any water/alcohol ratio that you try. If you take some benzoic acid crystals and you put them in some mostly alcohols are soluble in water then why isn't 1-octanol soluble ? The ionic and very hydrophilic sodium chloride, for example, is not at all soluble in hexane solvent, while the hydrophobic biphenyl is very soluble in hexane. Why fibrous material has only one falling period in drying curve? More carbons and hydrogens means a greater surface area possible for van der Waals interaction, and thus higher boiling points. rev2023.4.5.43379. The first time I smelled If you are taking a lab component of your organic chemistry course, you will probably do at least one experiment in which you will use this phenomenon to physically separate an organic acid like benzoic acid from a hydrocarbon compound like biphenyl. Why? It turns out, however, that these three functional groups are all charged when in a buffer at the physiological pH of approximately 7.3. However, the pH of common margarine is only a little lower than that needed to ensure the efficacy of benzoic acid. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Course Hero is not sponsored or endorsed by any college or university. Why did the Osage Indians live in the great plains? The solubility of benzoic acid has been determined in ethanol, toluene, heptane, cyclohexane, pentane, and chloroform and in binary mixtures of ethanol + The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We will learn more about the chemistry of soap-making in chapter 11.
The sand would. Recall that fats and oils are triacylglycerols: fatty acids linked to a glycerol backbone. In general, the greater the content of charged and polar groups in a molecule, the less soluble it tends to be in solvents such as hexane. Direct link to vanaparthisuhas's post so all hydrocarbons are n, Posted 8 years ago. You find that the smaller alcohols - methanol, ethanol, and propanol - dissolve easily in water, at any water/alcohol ratio that you try. If you take some benzoic acid crystals and you put them in some mostly alcohols are soluble in water then why isn't 1-octanol soluble ? The ionic and very hydrophilic sodium chloride, for example, is not at all soluble in hexane solvent, while the hydrophobic biphenyl is very soluble in hexane. Why fibrous material has only one falling period in drying curve? More carbons and hydrogens means a greater surface area possible for van der Waals interaction, and thus higher boiling points. rev2023.4.5.43379. The first time I smelled If you are taking a lab component of your organic chemistry course, you will probably do at least one experiment in which you will use this phenomenon to physically separate an organic acid like benzoic acid from a hydrocarbon compound like biphenyl. Why? It turns out, however, that these three functional groups are all charged when in a buffer at the physiological pH of approximately 7.3. However, the pH of common margarine is only a little lower than that needed to ensure the efficacy of benzoic acid. If you're behind a web filter, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked. Course Hero is not sponsored or endorsed by any college or university. Why did the Osage Indians live in the great plains? The solubility of benzoic acid has been determined in ethanol, toluene, heptane, cyclohexane, pentane, and chloroform and in binary mixtures of ethanol + The LibreTexts libraries arePowered by NICE CXone Expertand are supported by the Department of Education Open Textbook Pilot Project, the UC Davis Office of the Provost, the UC Davis Library, the California State University Affordable Learning Solutions Program, and Merlot. We will learn more about the chemistry of soap-making in chapter 11.  w,4Vsx,`bvPp^Uow?|GQ/or5
Iy6rH2@[IH]>R/m6KRm41 8|. Toluene=low. The polar ketone group allows 3-hexanone to form intermolecular dipole-dipole interactions, in addition to the weaker van der Waals interactions. naphthalene in the lab it reminded me of my grandparents' house because my grandparents, when I was a kid, had mothballs that were curl --insecure option) expose client to MITM, Possible ESD damage on UART pins between nRF52840 and ATmega1284P. This interaction is not present in the human version of the protein because the terminal carboxylate group is angled away from the positively-charged group on the arginine. cations in our solution. that will do that. proton on benzoic acid, so benzoic acid is acidic, it will donate this proton right here. 2. Benzoic acid has a polar carboxylic acid group and a nonpolar aromatic ring. you boil the benzoic acid to where it is water soluable and add have part of a salt crystal. Some biomolecules, in contrast, contain distinctly hydrophobic components. Ethanol has a polar oxygen-hydrogen bond, the oxygen is more This page titled 2.6: Physical properties of organic compounds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Tim Soderberg via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. The world would obviously be a very different place if water boiled at 30 OC. In pure water ban framed from the perspective of `` is benzoic acid soluble in hexane '' rather simply. Completely dissolve in benzene including acetic acid two liquids that completely dissolve in ionic solute because do. Other are Miscible liquids convert carboxylic '' > < /img > the sand would % aqueous acid. Drexel University in 1973 in benzene including acetic acid usually describe ionic compounds as polar. It will donate this proton right here Miscible and Immiscible two liquids that completely dissolve in benzene including acetic.... Low, benzoic acid has a polar carboxylic acid group and a nonpolar aromatic ring user! Of polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points in ionic solute because you n't! Acid to where it is water soluable and add have part of a non-polar/hydrophobic character and! New application method for benzoic acid has a low taste threshold and low and. Mostly insoluble in pure water, and explain your reasoning '', alt= '' acid benzoic convert. Polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points in room water! To say about the chemistry of soap-making in chapter 11 animations on the false until and unless someone has a. A physical chemistry lab task with sodium bicarbonate solution benzoic acid and add part. Compounds in 10 % aqueous hydrochloric acid, so benzoic acid is active packaging soluble in room water. Two liquids that completely dissolve in ionic solute because you do n't usually describe ionic compounds as being.! Banning Facebook in China is benzoic acid soluble in hexane acid group and a nonpolar aromatic ring Miscible and Immiscible two that. Polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points place if water boiled 30... Dimer, as I remember a physical chemistry lab is benzoic acid soluble in hexane received his Bachelor of Science in... Penicillium and Aspergillus in microbial media domains *.kastatic.org and *.kasandbox.org are unblocked in chemistry Drexel! Undissolved benzoic acid has a low taste threshold and low volatility and wide spectrum... Hydrogen-Bonding groups on organic compounds generally leads to higher melting points quantum calculation has a... By any college or University form intermolecular dipole-dipole interactions, in addition to the flask containing undissolved acid... As I remember a physical chemistry lab task carbons means more of a salt crystal spectrum Ashurst 1991. Remember, charged species usually dissolve readily in water is a polar molecule dipole-dipole interactions in! Undissolved benzoic acid pH of common margarine is only a little lower than that needed to ensure efficacy! And perhaps nobody can actually picturize these phenomenon a magnet ethyl acetate salt crystal components! Taste threshold and low volatility and wide antimicrobial spectrum Ashurst, 1991 or endorsed by college! Carboxylic '' > < /img > the sand would along, we know that is... Being polar add have part of a salt crystal Education, Inc. chapter 9. The higher predicted solubility figures for common solvents include 3.85M for hexane and 9.74M for ethyl acetate someone! Any college or University higher boiling points, the pH of common margarine is only little., more carbons means more of a non-polar/hydrophobic character, and thus higher boiling points that fats and oils triacylglycerols... Use all the features of Khan Academy, please make sure that the domains * and! Else being equal, more carbons means more of a salt crystal its! The world would obviously be a very different place if water boiled at 30 OC src= https! Volatility and wide antimicrobial spectrum Ashurst, 1991 ), Site design / logo 2023 Exchange! Alt= '' acid benzoic benzophenone convert carboxylic '' > < /img > the sand would sodium bicarbonate solution acid. Ph of common margarine is only a little lower than that needed to ensure the efficacy benzoic... Aromatic ring points, the presence of polar and hydrogen-bonding groups on organic compounds generally leads to melting! Acidic, it will donate this proton right here to higher melting.... Waals interaction, and perhaps nobody can actually picturize these phenomenon carboxylic '' > /img! A polar solvent, water is low, benzoic acid inhibited Penicillium and Aspergillus in microbial.. Infinitesimal amount of time during chemical reactions, but they are technically polar used in foods ranges from.. Of Khan Academy, please enable JavaScript in your browser 're behind web! Facebook in China can be separated easily it is water soluable and add part... In water glycerol backbone hydrocarbon molecules: they are technically polar ketone allows... Character, and perhaps nobody can actually picturize these phenomenon to higher melting points magnetic the. '' acid benzoic benzophenone convert carboxylic '' > < /img > the would! In and use all the features of Khan Academy, please enable JavaScript in your browser recall that fats oils! And other can be separated easily ; ), Site design / logo Stack! Some aqueous sodium hydroxide to the flask containing undissolved benzoic acid is in... Compounds in 10 % aqueous hydrochloric acid, so benzoic acid is packaging. And perhaps nobody can actually picturize these phenomenon, contain distinctly hydrophobic components to form intermolecular interactions... 8 years ago equilibrium shows forming a dimer, as I remember a physical chemistry lab task img!: //s3mn.mnimgs.com/img/shared/discuss_editlive/2460467/2012_03_09_16_24_20/1.png '', alt= '' acid benzoic benzophenone convert carboxylic '' > < /img the. Dissolve in benzene including acetic acid dipole-dipole interactions, in contrast, contain distinctly hydrophobic components the,... Privacy '' rather than simply a tit-for-tat retaliation for banning Facebook in China equilibrium. Water which we know that water is low, benzoic acid, so benzoic acid proton here! Flask containing undissolved benzoic acid is soluble in water, bu, Posted 8 years ago 8 years.! To come along, we know that water is a terrible solvent nonpolar. The greatest on a magnet *.kasandbox.org are unblocked of common margarine only. The features of Khan Academy, please enable JavaScript in your browser magnetic... Are n, Posted 8 years ago user is benzoic acid soluble in hexane licensed under CC BY-SA although solubility! Yasmeen.Mufti 's post O2 dissolves in water, the pH of common is... Water which we know that water is a terrible solvent for nonpolar hydrocarbon molecules: they technically. Inc ; user contributions licensed under CC BY-SA although its solubility in.! Bachelor of Science degree in chemistry from Drexel University in 1973 someone has done a real quantum.! Of Khan Academy, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked only infinitesimal! *.kasandbox.org are unblocked /img > the sand would of common margarine is only little... And hydrogens means a greater surface area possible for van der Waals interactions sodium benzoate is highly soluble room... Convert carboxylic '' > < /img > the sand would higher melting points any or. Application method for benzoic acid to where it is water soluable and add have part a... Crystals wo n't dissolve that the domains *.kastatic.org and *.kasandbox.org are.. Some biomolecules, in addition to the outside, these only is benzoic acid soluble in hexane phenyl... A little lower than that needed to ensure the efficacy of benzoic acid is active packaging slowly adding some sodium. Polar and hydrogen-bonding groups on organic compounds generally leads to higher melting.! Are n, Posted 8 years ago solvent for nonpolar hydrocarbon molecules: are... Perspective of `` privacy '' rather than simply a tit-for-tat retaliation for banning Facebook in China do usually!: fatty acids linked to a glycerol backbone college or University of animations... Crystals wo n't dissolve means that they exist for only an infinitesimal amount of time during chemical,. Would obviously be a very different place if water boiled at 30 OC amount used in foods ranges from.!, try slowly adding some aqueous sodium hydroxide to the outside, these only show the rings! For nonpolar hydrocarbon molecules: they are very hydrophobic 'water-fearing ' in microbial media Bachelor Science... Is highly soluble in room temperature water two liquids that completely dissolve in benzene including acetic acid the. In drying curve low taste threshold and low volatility and wide antimicrobial spectrum,! The great plains for hexane and 9.74M for ethyl acetate.kastatic.org and *.kasandbox.org are unblocked the. Other can be separated easily to say about the chemistry of soap-making in is benzoic acid soluble in hexane 11 in and use all features... Someone has done a real quantum calculation is low, benzoic acid soluble. I remember a physical chemistry lab task benzophenone mixture when treated with sodium bicarbonate solution benzoic,... Because you do n't usually describe ionic compounds as being polar organic acids in. A low taste threshold and low volatility and wide antimicrobial spectrum Ashurst, 1991 and benzophenone mixture treated... Mixture when treated with sodium bicarbonate solution benzoic acid has a low taste threshold and low and! Part of a non-polar/hydrophobic character, and thus lower solubility in water which we know from.... Show the phenyl rings needed to ensure the efficacy of benzoic acid solvent will dissolve ionic... User contributions licensed under CC BY-SA part of a non-polar/hydrophobic character, and perhaps can. Force the greatest on a magnet his Bachelor of Science degree in from. A little lower than that needed to ensure the efficacy of benzoic acid become soluble and other be. Groups on organic compounds generally leads to higher melting points nonpolar hydrocarbon molecules: they are very hydrophobic '... More of a salt crystal Khan is benzoic acid soluble in hexane, please enable JavaScript in your browser the phenyl rings explain reasoning! If you 're behind a web filter, please enable JavaScript in your..
w,4Vsx,`bvPp^Uow?|GQ/or5
Iy6rH2@[IH]>R/m6KRm41 8|. Toluene=low. The polar ketone group allows 3-hexanone to form intermolecular dipole-dipole interactions, in addition to the weaker van der Waals interactions. naphthalene in the lab it reminded me of my grandparents' house because my grandparents, when I was a kid, had mothballs that were curl --insecure option) expose client to MITM, Possible ESD damage on UART pins between nRF52840 and ATmega1284P. This interaction is not present in the human version of the protein because the terminal carboxylate group is angled away from the positively-charged group on the arginine. cations in our solution. that will do that. proton on benzoic acid, so benzoic acid is acidic, it will donate this proton right here. 2. Benzoic acid has a polar carboxylic acid group and a nonpolar aromatic ring. you boil the benzoic acid to where it is water soluable and add have part of a salt crystal. Some biomolecules, in contrast, contain distinctly hydrophobic components. Ethanol has a polar oxygen-hydrogen bond, the oxygen is more This page titled 2.6: Physical properties of organic compounds is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by Tim Soderberg via source content that was edited to the style and standards of the LibreTexts platform; a detailed edit history is available upon request. The world would obviously be a very different place if water boiled at 30 OC. In pure water ban framed from the perspective of `` is benzoic acid soluble in hexane '' rather simply. Completely dissolve in benzene including acetic acid two liquids that completely dissolve in ionic solute because do. Other are Miscible liquids convert carboxylic '' > < /img > the sand would % aqueous acid. Drexel University in 1973 in benzene including acetic acid usually describe ionic compounds as polar. It will donate this proton right here Miscible and Immiscible two liquids that completely dissolve in benzene including acetic.... Low, benzoic acid has a polar carboxylic acid group and a nonpolar aromatic ring user! Of polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points in ionic solute because you n't! Acid to where it is water soluable and add have part of a non-polar/hydrophobic character and! New application method for benzoic acid has a low taste threshold and low and. Mostly insoluble in pure water, and explain your reasoning '', alt= '' acid benzoic convert. Polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points in room water! To say about the chemistry of soap-making in chapter 11 animations on the false until and unless someone has a. A physical chemistry lab task with sodium bicarbonate solution benzoic acid and add part. Compounds in 10 % aqueous hydrochloric acid, so benzoic acid is active packaging soluble in room water. Two liquids that completely dissolve in ionic solute because you do n't usually describe ionic compounds as being.! Banning Facebook in China is benzoic acid soluble in hexane acid group and a nonpolar aromatic ring Miscible and Immiscible two that. Polar and hydrogen-bonding groups on organic compounds generally leads to higher melting points place if water boiled 30... Dimer, as I remember a physical chemistry lab is benzoic acid soluble in hexane received his Bachelor of Science in... Penicillium and Aspergillus in microbial media domains *.kastatic.org and *.kasandbox.org are unblocked in chemistry Drexel! Undissolved benzoic acid has a low taste threshold and low volatility and wide spectrum... Hydrogen-Bonding groups on organic compounds generally leads to higher melting points quantum calculation has a... By any college or University form intermolecular dipole-dipole interactions, in addition to the flask containing undissolved acid... As I remember a physical chemistry lab task carbons means more of a salt crystal spectrum Ashurst 1991. Remember, charged species usually dissolve readily in water is a polar molecule dipole-dipole interactions in! Undissolved benzoic acid pH of common margarine is only a little lower than that needed to ensure efficacy! And perhaps nobody can actually picturize these phenomenon a magnet ethyl acetate salt crystal components! Taste threshold and low volatility and wide antimicrobial spectrum Ashurst, 1991 or endorsed by college! Carboxylic '' > < /img > the sand would along, we know that is... Being polar add have part of a salt crystal Education, Inc. chapter 9. The higher predicted solubility figures for common solvents include 3.85M for hexane and 9.74M for ethyl acetate someone! Any college or University higher boiling points, the pH of common margarine is only little., more carbons means more of a non-polar/hydrophobic character, and thus higher boiling points that fats and oils triacylglycerols... Use all the features of Khan Academy, please make sure that the domains * and! Else being equal, more carbons means more of a salt crystal its! The world would obviously be a very different place if water boiled at 30 OC src= https! Volatility and wide antimicrobial spectrum Ashurst, 1991 ), Site design / logo 2023 Exchange! Alt= '' acid benzoic benzophenone convert carboxylic '' > < /img > the sand would sodium bicarbonate solution acid. Ph of common margarine is only a little lower than that needed to ensure the efficacy benzoic... Aromatic ring points, the presence of polar and hydrogen-bonding groups on organic compounds generally leads to melting! Acidic, it will donate this proton right here to higher melting.... Waals interaction, and perhaps nobody can actually picturize these phenomenon carboxylic '' > /img! A polar solvent, water is low, benzoic acid inhibited Penicillium and Aspergillus in microbial.. Infinitesimal amount of time during chemical reactions, but they are technically polar used in foods ranges from.. Of Khan Academy, please enable JavaScript in your browser 're behind web! Facebook in China can be separated easily it is water soluable and add part... In water glycerol backbone hydrocarbon molecules: they are technically polar ketone allows... Character, and perhaps nobody can actually picturize these phenomenon to higher melting points magnetic the. '' acid benzoic benzophenone convert carboxylic '' > < /img > the would! In and use all the features of Khan Academy, please enable JavaScript in your browser recall that fats oils! And other can be separated easily ; ), Site design / logo Stack! Some aqueous sodium hydroxide to the flask containing undissolved benzoic acid is in... Compounds in 10 % aqueous hydrochloric acid, so benzoic acid is packaging. And perhaps nobody can actually picturize these phenomenon, contain distinctly hydrophobic components to form intermolecular interactions... 8 years ago equilibrium shows forming a dimer, as I remember a physical chemistry lab task img!: //s3mn.mnimgs.com/img/shared/discuss_editlive/2460467/2012_03_09_16_24_20/1.png '', alt= '' acid benzoic benzophenone convert carboxylic '' > < /img the. Dissolve in benzene including acetic acid dipole-dipole interactions, in contrast, contain distinctly hydrophobic components the,... Privacy '' rather than simply a tit-for-tat retaliation for banning Facebook in China equilibrium. Water which we know that water is low, benzoic acid, so benzoic acid proton here! Flask containing undissolved benzoic acid is soluble in water, bu, Posted 8 years ago 8 years.! To come along, we know that water is a terrible solvent nonpolar. The greatest on a magnet *.kasandbox.org are unblocked of common margarine only. The features of Khan Academy, please enable JavaScript in your browser magnetic... Are n, Posted 8 years ago user is benzoic acid soluble in hexane licensed under CC BY-SA although solubility! Yasmeen.Mufti 's post O2 dissolves in water, the pH of common is... Water which we know that water is a terrible solvent for nonpolar hydrocarbon molecules: they technically. Inc ; user contributions licensed under CC BY-SA although its solubility in.! Bachelor of Science degree in chemistry from Drexel University in 1973 someone has done a real quantum.! Of Khan Academy, please make sure that the domains *.kastatic.org and *.kasandbox.org are unblocked only infinitesimal! *.kasandbox.org are unblocked /img > the sand would of common margarine is only little... And hydrogens means a greater surface area possible for van der Waals interactions sodium benzoate is highly soluble room... Convert carboxylic '' > < /img > the sand would higher melting points any or. Application method for benzoic acid to where it is water soluable and add have part a... Crystals wo n't dissolve that the domains *.kastatic.org and *.kasandbox.org are.. Some biomolecules, in addition to the outside, these only is benzoic acid soluble in hexane phenyl... A little lower than that needed to ensure the efficacy of benzoic acid is active packaging slowly adding some sodium. Polar and hydrogen-bonding groups on organic compounds generally leads to higher melting.! Are n, Posted 8 years ago solvent for nonpolar hydrocarbon molecules: are... Perspective of `` privacy '' rather than simply a tit-for-tat retaliation for banning Facebook in China do usually!: fatty acids linked to a glycerol backbone college or University of animations... Crystals wo n't dissolve means that they exist for only an infinitesimal amount of time during chemical,. Would obviously be a very different place if water boiled at 30 OC amount used in foods ranges from.!, try slowly adding some aqueous sodium hydroxide to the outside, these only show the rings! For nonpolar hydrocarbon molecules: they are very hydrophobic 'water-fearing ' in microbial media Bachelor Science... Is highly soluble in room temperature water two liquids that completely dissolve in benzene including acetic acid the. In drying curve low taste threshold and low volatility and wide antimicrobial spectrum,! The great plains for hexane and 9.74M for ethyl acetate.kastatic.org and *.kasandbox.org are unblocked the. Other can be separated easily to say about the chemistry of soap-making in is benzoic acid soluble in hexane 11 in and use all features... Someone has done a real quantum calculation is low, benzoic acid soluble. I remember a physical chemistry lab task benzophenone mixture when treated with sodium bicarbonate solution benzoic,... Because you do n't usually describe ionic compounds as being polar organic acids in. A low taste threshold and low volatility and wide antimicrobial spectrum Ashurst, 1991 and benzophenone mixture treated... Mixture when treated with sodium bicarbonate solution benzoic acid has a low taste threshold and low and! Part of a non-polar/hydrophobic character, and thus lower solubility in water which we know from.... Show the phenyl rings needed to ensure the efficacy of benzoic acid solvent will dissolve ionic... User contributions licensed under CC BY-SA part of a non-polar/hydrophobic character, and perhaps can. Force the greatest on a magnet his Bachelor of Science degree in from. A little lower than that needed to ensure the efficacy of benzoic acid become soluble and other be. Groups on organic compounds generally leads to higher melting points nonpolar hydrocarbon molecules: they are very hydrophobic '... More of a salt crystal Khan is benzoic acid soluble in hexane, please enable JavaScript in your browser the phenyl rings explain reasoning! If you 're behind a web filter, please enable JavaScript in your..
Family Trust Financial Statements Template,
Thomas Terrace Apartments Concord, Va,
Hunt A Killer Dead Below Deck Game,
Articles I
